Antimycotic Susceptibility Testing (Candida)
Product Details:
Antimycotic Susceptibility Testing (Candida) Price And Quantity
- 4350.00 - 10800.00 INR
- 1 Box
- 4350 INR
Product Description
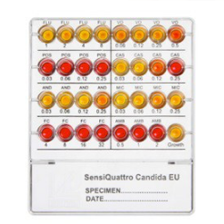
SensiQuattro Candida EU
Antimycotic Susceptibility Testing with Liofilchem® SensiQuattro Candida EU
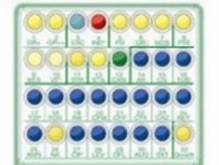
The new Liofilchem® SensiQuattro Candida EU is a novel 32-well panel containing 8 desiccated antimycotics* at four doubling serial concentrations that allows determination of susceptibility of Candida spp. to antimycotic agents.
* Fluconazole, Voriconazole, Posaconazole, Caspofungin, Anidulafungin, Micafungin, Flucytosine, Amphotericin B.
The results, when correlated with EUCAST (The European Committee on Antimicrobial Susceptibility Testing) breakpoints permit accurate determination of the microorganism as Sensitive (S) or Resistant (R) to the antimycotics.
The panel is inoculated and rehydrated with a standardised microbial suspension then incubated at 34.5-35.5 °C for 24±2 hours. After incubation, the susceptibility of Candida spp. to the antimycotic agents is assessed on the basis of growth or inhibition of the microorganisms in media containing antimycotic and a growth indicator that causes color change.
Susceptibility testing results provided by SensiQuattro Candida EU must be evaluated according to EUCAST clinical breakpoints and the microorganisms can be interpreted as S (Sensitive) or R (Resistant).
SensiQuattro di Liofilchem
Liofilchem strengthens its position as a leader in manufacturing reagents for the antimicrobial susceptibility testing, adding the SensiQuattro Candida EU to its ranges of MIC Test Strip, antibiotic and antifungal disks, dehydrated and ready to use culture media.
REFERENCES
- EUCAST. Antifungal Agents Breakpoint tables for interpretation of MICs. Version 5.0, January 2013.
- EUCAST Definitive Document EDef7.1:method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Subcommittee on antifungal Susceptibility testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Clin Microbiol Infect 2008; 14: 398-405.
- Multicentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). M.Cuenca-Estrella et al. Clin Microbiol infect 2007; 13:1018-1022.
Contacts
Liofilchem, Inc.
465 Waverley Oaks Rd. Suite 317
Waltham, MA 02452

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Micro biology product' category
 |
SR GROUP
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |