COVID-19 Real-Time PCR Kit
Product Details:
COVID-19 Real-Time PCR Kit Price And Quantity
- 1.00 - 5.00 INR/Pack
- 75000-90000 INR/Pack
- 1 Kit
COVID-19 Real-Time PCR Kit Trade Information
- NEW DELHI
- Cash Against Delivery (CAD)
- 100 Kit Per Day
- 10 Days
- Yes
- Free samples are available
- 100 TEST
- Asia
- All India
- USFDA
Product Description
The Allplex 2019-nCoV Assay is an in vitro diagnostic (IVD) real-time reverse transcriptase polymerase chain reaction (RT-PCR) test intended for the qualitative detection ofnucleic acid from severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) in human nasopharyngeal swab, oropharyngeal swab, anterior nasal swab, midturbinate and sputum specimens from individuals with signs and symptoms of infection who are suspected of COVID-19 by their health care provider
Expiry date is 8 months from the date of manufacture at -20.Please refer to product label for final expiry date. This product can be used for 30 days after opening the vials. This product can be used for maximum & repeats of freezing and thawing.
CMR APPROVED CE IVD CERTIFIED
AllplexTM 2019-nCoV Assay is in vitro diagnostic medical device designed for qualitative detection of novel Corona virus (2019-nCoV) with
real-time reverse transcription PCR from sputum, nasopharyngeal aspirate, throat & nasopharyngeal swab, and bronchoalveolar lavage.
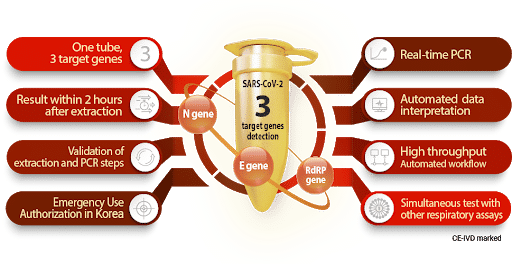
3 target genes detection
Allplex 2019-nCoV Assay is multiplex Real-time PCR assay for simultaneous detection of 3 target genes of SARS-CoV-2 in a single tube. The assay is designed to detect RdRP and N genes specific for SARS-CoV-2, and E gene for all of Sarbecovirus including SARS-CoV-2
Specimens
- Nasopharyngeal swab
- Nasopharyngeal aspirate
- Throat swab
- Bronchoalveolar lavage
- Sputum

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'Micro biology product' category
 |
SR GROUP
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |